The field of stem cell (SC) research is evolving rapidly. There are over 600 neurodegenerative diseases, each with its unique set of symptoms, progression and underlying pathology.
In February 2024 scientists researching stem cells at the Singapore-MIT Alliance for Research and Technology (SMART) announced the development of a tool to create an in-vitro model of a neurodegenerative disease – this may represent a significant step forward for neurodegenerative disease therapy development.
The SMART team tool is called a microfluidic device. Microfluidic devices are systems that manipulates fluids on a microscopic scale. The devices are made up of structures moulded into a substrate, e.g. glass, silicon or polymer.
Microfluidic devices create in-vitro models of neurodegenerative diseases by culturing stem cells and induced pluripotent stem cells (iPSCs) in controlled microenvironments. The SMART team’s microfluidic devices enable stem cells to differentiate into specific neural cell types.
Microfluidic devices aim to make cell therapies safer for patients and deliver them at a lower cost. The tiny microfluidic chip is able to sort cells by size, for example, removing cells that have not yet become spinal cord cells – such undifferentiated cells have the potential to form tumours after a transplant.
- The use of undifferentiated induced pluripotent stem cells (iPSCs) carries a high risk of tumour formation. iPSCs can differentiate into any cell type, but if they remain undifferentiated they can proliferate uncontrollably, and then can form teratomas and other tumours. (Pluripotent means a cell has the ability to differentiate into various types of cells.
- Microfluidic devices can sort ~3m cells/min and filter out ‘risky’ cells that could potentially become tumours when implanted in a patient. An additional cost and safety advantage is that microfluidic devices do not require specialty chemicals to operate.
- According to Singapore-MIT (SMART) researchers microfluidic devices can be chained together to sort over 500m cells/min. It is possible that the process could allow stem differentiation to become a ‘factory’ scale process, which again has potentially significant cost saving implications.
Exhibit 1 – Microfluidic device
 Sources: Wikimedia Commons.
Sources: Wikimedia Commons.
The stem cell market’s value is expected to reach US$ 30bn by 2030E
Stem cell therapy can be administered through various routes, including intravenous infusion, intrathecal injection and direct implantation into the brain or spinal cord. There are over 2,000 clinical trials underway globally to explore the potential of stem cell therapies for various diseases, including neurodegenerative disorders, for example, BrainStorm Cell Therapeutics’ (Nasdaq;BCLI) approach to motor neurone disease (MND), also referred to as ALS or Lou Gehrig’s disease or amyotrophic lateral sclerosis (ALS).
Clinical trials are providing some promising results for the improvement of motor function, cognitive abilities and so possibly quality-adjusted life years (QALY) in patients with neurodegenerative diseases such as Parkinson’s and Alzheimer’s.
Even in modern medicine treating neurodegenerative diseases is, currently, still extremely challenging and most likely requires a multidiscipline effort. The integration of microfluidic devices, stem cell technology and research on neurodegenerative diseases represents just such a multidisciplinary effort. Collectively these disciplines attempt to address disease mechanisms, develop novel treatments and advance regenerative medicine. The ultimate aim is to deliver personalised therapies that combat neurological disorders.
Neurodegenerative diseases such as Alzheimer, Parkinson’s and related disorders affect ~60m people worldwide – this number is expected to double by 2050E (JPND Research, 2023 & WHO, 2023). Therefore, any potential treatment resulting from advancements in stem cell research offers a glimpse of hope.
The global stem cell (SC) therapy market is expected to reach a value of ~ US$ 31.41bn by 2030E, up from US$ 11.8bn in 2022A (Biospace, 2023 & Global Markets Insights, 2023). The main drivers for the expected growth in the value of the stem call market are increasing prevalence of chronic diseases, technological advancements and accelerated funding.
The cost of stem cell therapy varies widely depending on the type of treatment and geography. The average cost range may be anything from $5,000 to $30,000 per treatment session (BioInformant, elclinics, 2024).
Despite the high cost of stem cell therapy, the long-term benefits compensate for the initial high costs. If widely accessible, stem cell therapy could potentially reduce healthcare expenditures associated with prolonged care and management of symptoms for chronic, long term, disease states.
Because of the complexities of neurodegenerative diseases, stem cells perhaps represent the therapy set with the most transformational QALY potential. The future for stem cells, in our view, remains very bright.
Stem cell therapy applications – bywords for flexibility and versatility
The versatility of stem cells offers flexibility in treatment approaches – we describe the six categories of stem cells below:
- Embryonic Stem Cells (ESCs) – Pluripotent cells derived from the inner cell mass of early embryos. ESCs can differentiate into any cell type on the body. Researchers are exploring ESC potential for treating neurological diseases (amongst others).
- Tissue-Specific Progenitor Stem Cells (TSPSCs) – Multipotent cells (they can only differentiate into a limited range of cell types) found in tissues such as bone marrow and the brain. TSPSCs offer targeted therapies for tissue repair and regeneration.
- Mesenchymal Stem Cells (MSCs) – Multipotent cells found in bone marrow and adipose tissue (specialised connective tissue composed of fat cells – adipocytes). MSCs are used in tissue repair and immunomodulation (modifying/regulating immune responses), e.g. BCLI’s ALS/MND P3b planned trial.
- Umbilical Cord Stem Cells (UCSCs) – Cells derived from the blood and tissue of the umbilical cord after childbirth. UCSCs are prized for their low risk of rejection and versatility in regenerative medicine for blood disorders, e.g. leukaemia and lymphoma.
- Bone Marrow Stem Cells (BMSCs) – Multipotent cells found in bone marrow. BMSCs are valued for their ability to differentiate into red and white blood cells and are used in bone marrow transplants to treat blood disorders, e.g. leukaemia and lymphoma.
- Induced Pluripotent Stem Cells (iPSCs) – Cells derived from adult cells, which can also be generated from a patient’s own cells. iPSCs reduce the risk of immune rejection and have a potentially unlimited supply of therapeutic applications.
Dependent on the category of stem cells, therapies for neurodegenerative disorders can be administrated using a range of approaches from injections to infusions to direct implantation of the stem cells into a patient’s brain or spinal cord.
The ultimate paradigm is that stem cell therapies replace damaged cells, regenerate neural networks and restore functions in affected individuals. Investors should note that significant concerns over both safety and efficacy of such interventions have not yet been entirely addressed.
Nevertheless, current stem cell therapy clinical trials are showing promising results that range from improving motor function to some restoration of cognitive abilities, thereby delivering an improved QALY for patients with neurodegenerative diseases such as Parkinson’s, Alzheimer’s and Lou Gehrig’s (MND/ALS).
There are approximately 250 companies globally that are focusing on stem cell therapy. Amongst these are Vertex Pharmaceuticals Inc. (Nasdaq: VRTX), Vericel Corporation (Nasdaq: VCEL), Sangamo Therapeutics Inc. (Nasdaq: SGMO), Brainstorm Cell Therapeutics Inc. (Nasdaq: BCLI) and Gamida Cell Ltd. (Nasdaq: GMDA) as per exhibit 1 below.
Exhibit 2 – Peer group of companies focused on stem cell therapy as on 16 Apr 2024
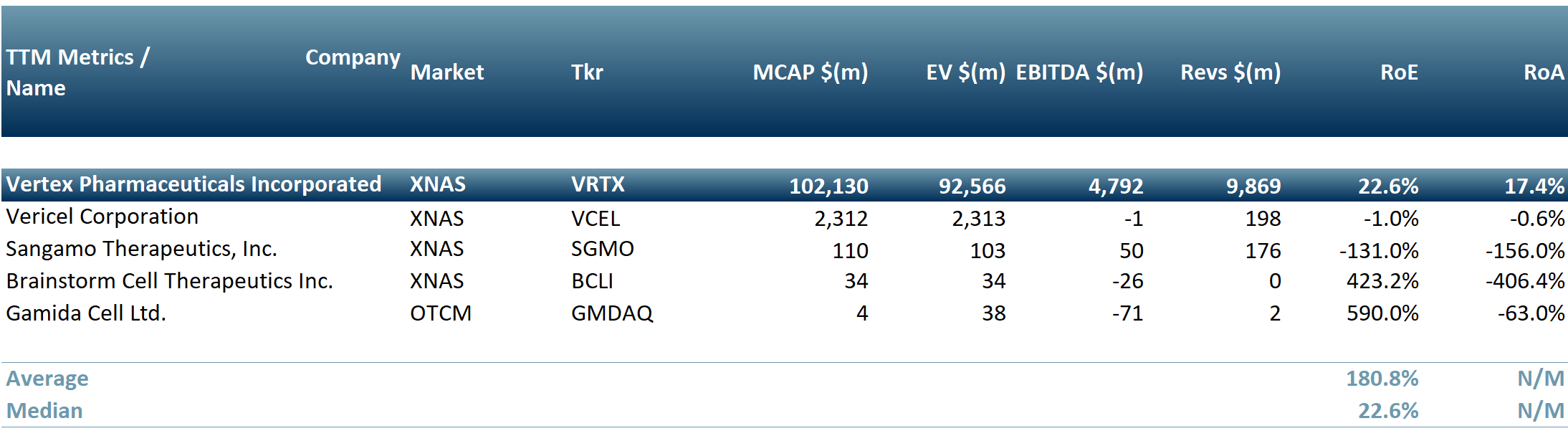 Sources: ACF Equity Research Graphics; Refinitiv
Sources: ACF Equity Research Graphics; Refinitiv




















