Long Covid – the new chronic condition?
Researchers estimate that globally there are over 200m people with long Covid, with the duration of this condition varying from months to years. In Europe there are ~17m cases, in the US ~16m and in the UK ~2m.
To date there are ~26 clinical trials investigating long Covid therapies. These suggest the issue is in hand, but according to immunologist Danny Altmann at Imperial College London, long Covid presently looks like the “Wild West” and paints a “desperate picture really”.
Nevertheless, researchers are optimistic. Some trials are testing ‘familiar’ therapies/drugs such as anti-inflammatory, dietary supplements, cell-based therapy, steroids and antithrombotics. Others are looking to experimental drugs or even cognitive therapy to ‘manage’ certain symptoms. Antivirals are also subject to investigation.
There are, simultaneously, many independent trials and studies underway, though some are yet to enrol patients. The pandemic has revealed just how effectively a collective constructive approach can be to problem solving in healthcare. This collective approach has also generated significant investor value. But it should be remembered that this has happened through the cooperation between, companies, markets and governments.
- The UK initiated a study in August 2021 for its STIMULATE-ICP (Symptoms, Trajectory, Inequalities and Management: Understanding Long-Covid to Address and Transform Existing Integrated Care Pathways).
- The study began enrolling patients in the summer of 2022 – 4,500 individuals with long Covid. (Nature, 2022)
- The trial will test various medications in the hope of combatting long Covid beginning with anti-inflammatory colchicine, two antihistamines (famotidine and loratadine) and an anticlotting drug (rivaroxaban). (STIMULATE-ICP, 2022)
- Antivirals are under investigation, however there are no registered studies. Two antivirals have been approved by the US Food and Drug Administration (FDA): Merck’s molnupiravir (Lagevrio) and Pfizer’s nirmatrelvir and ritonavir (Paxlovid) combo, of which an indirect assessment is expected later this year.
Long Covid is very complex
Global health organisations (e.g. WHO, CDC and NIH) define long Covid as anywhere from symptoms persisting 4+ weeks after infection to 12 weeks. Long Covid is primarily diagnosed by investigating its ‘constellation’ of symptoms that are otherwise unexplained by an alternative diagnosis.
More than 200 symptoms have been identified and associated with long Covid. These include (but are not limited to) fatigue, muscle weakness, shortness of breath, gastrointestinal symptoms, blood clotting, memory loss and concentration difficulties.
In addition, stress-related mental disorders (e.g. major depressive disorder) increased during the pandemic, making the diagnosis more complex.
According to a study published in The Lancet medical journal in August 2022 using data from the Netherlands, 1 in 8 people experience long Covid after coronavirus infection. The CDC also released a study that indicated there is an increased risk of rare and serious complications in children that have been infected by Covid. (Bloomberg, 2022)
The data shows just how urgent (and complex) finding a solution for long Covid is, especially with the ongoing mutations of Sars-CoV2. It is therefore timely to investigate treatment alternatives that go beyond the vaccine.
Government funding – is it the way forward and should it be entirely relied upon?
• To date, the UK’s National Health System (NHS) has invested over £50m for 19 long Covid research projects. These projects examine symptoms and test possible treatments. These projects also explore the usefulness of specialist clinics and how these clinics can meet individual’s needs.
• In May 2022, the European Commission made a grant to the HUS Helsinki University Hospital of €6.55m (~£5.72m). The HUS consortium will investigate mechanisms and biomarkers of long-term effects of Covid and rehabilitation. The investigation findings will be used to treat patients at HUS and internationally.
• In March 2021 the US’ National Institutes of Health (NIH) announced a $1.15bn (~£1bn) 4-year initiative to investigate who develops long Covid and who does not, amongst all age cohorts. The US considers long-term tracking (longitudinal studies) necessary to provide a full picture.
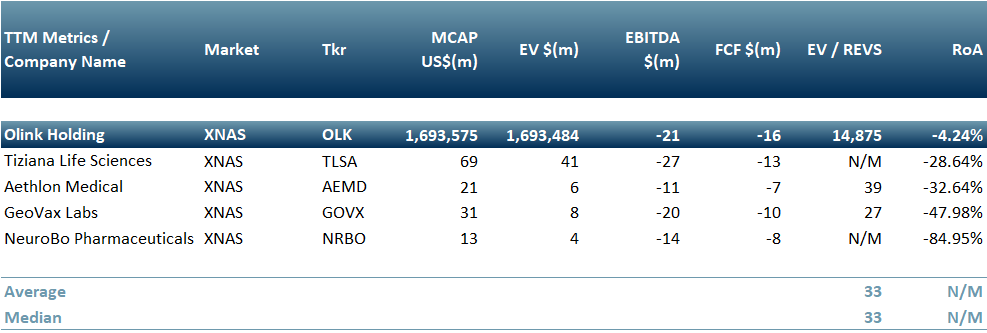
In Exhibit 1 we present pharma companies that are already testing or planning to test a treatment for long Covid: Geovax (Nasdaq: GOVX), Neurobo Pharmaceuticals (Nasdaq: NRBO), Tiziana Life Sciences (Nasdaq: TLSA), Aethlon Medical (Nasdaq: AEMD) and Olink Holding (Nasdaq: OLK).
Our inference is that this is a potentially attractive and long term revenue generating opportunity for these peers – however it is also a high risk enterprise due to complexity and in some cases may turn out to be a low RoI diversion, as is the nature of markets.
(There is lower risk associated with companies looking for a secondary indication for therapies either in development or already revenue generating).
Exhibit 1 – Peer Group on companies looking into long covid treatments
To know more about our Services: click here to see our entry point products and services