Trials of a vaccine and a new drug raise hopes of beating Covid-19
Oxford University published a paper in the Lancet on their trial of a Covid-19 vaccine on the 20th of July 2020. Even though Oxford University has been researching a vaccine since January 2020 and AstraZeneca (AZN.L) rushed to get one into production, there are still concerns around the safety and ability that the vaccines will actually work. The university believes that some of these safety and efficacy concerns have been resolved.
The trial involved 1,000 volunteers.
AstraZeneca plans billions of doses for global distribution.
According to Adrian Hill, director of Oxford’s Jenner Institute, ‘the Oxford vaccine stimulated a strong immune response and appears to be well tolerated and safe as it generated antibodies and “an excellent” T-cell response’.
Antibodies will destroy foreign bacteria/viruses (antigens) while T-cells will neutralise already infected cells to stop bacteria/viruses from proliferating further.
A study from Duke-NUS Medical School in Singapore observed that the T-cells of patients that were infected with SARS retained some ‘memory’ of the virus therefore, easing concerns that if antibodies do not work sufficiently T-cells can.
Encouraging results have been reported from the early stage of four vaccine trials in addition to the data previously published by AstraZeneca (AZN), Moderna (MRNA), Pfizer (PFE)-BioNTech (BNTX) and CanSino Biologics (6185.HK), a Chinese company that also made their announcement on 20th July.
Synairgen (SNG.L), a small-cap British biotech company, also announced on 20th July that it might have developed a possible drug treatment for Covid-19 in the form of inhaled interferon beta.
When using Synairgen’s inhaled interferon beta, the number of patients requiring ventilation fell by 79% and the rate of recovery increased by 2-3x.
The drug was only tested on 100 individuals and its findings have yet to be reviewed. Nevertheless, it provides a glimmer of hope that a curative (as opposed to just the vaccine strategy) for Covid can also be found.
Exhibit 1 below lists eight companies that are currently carrying out clinical trials for a Covid-19 vaccine that have positive results. The table illustrates that we may be well on our way to finding a vaccine as six out of the eight are entering or are already in Phase III (PIII) clinical trials.
Exhibit 1 – Covid-19 vaccines and treatments, 2020
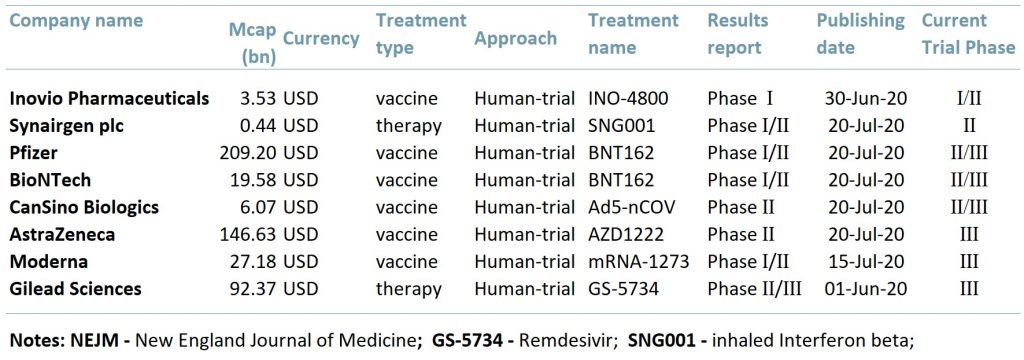
ACF view: There are 160 possible vaccines being produced globally, however only about 25 are undergoing human clinical trials. The recent positive results from the trials increase optimism that we may have a successful vaccine soon and also further verifies the V-shape economic recovery forecast.
The only way to fight this pandemic is via a vaccine. The amount of vaccines being produced shows how the healthcare sector constituent companies can work together for a common cause when appropriate funding or incentives are made available.
Potential new Covid-19 treatments that are being produced by small-cap companies such as Synairgen, could stimulate further competition for ‘big pharma’ products e.g. Gilead’s (GILD) Remdesivir treatment indication for Covid-19. Small and mid-cap companies generate the most jobs. These businesses need the support from private and public entities in order to thrive, which they are proving that they can.
As we embark on this next phase of Covid-19, access to funding is key along with an even playing field in capital markets in order to bring about the hoped end-result – finding a vaccine as soon as possible.
















