A new era in cancer diagnosis
In June 21, Grail (a healthcare company) released its cancer diagnosis blood test. This has the potential to change cancer diagnosis over the next 5 years.
Key Points:
- Grail, a VC backed private healthcare company, has launched its Galleri blood test. This is a ground-breaking multi-cancer screening tool used for early diagnosis of >50 forms of cancer.
- The latest study involved 4,077 people, 2,832 of which had previously been diagnosed with cancer. It showed that Grail’s Galleri test could correctly identify the presence of cancer in 51.5% of cases.
- This is achieved by decoding fragments of tumour DNA that are found in the bloodstream.
- The test was made available by prescription in the US in early June after years of development, US$ ~2bn in venture capital funds, and clinical research programs spanning over 115k patients.
- The UK’s National Health Service (NHS) will launch a pilot program starting in 2022 to screen at least 140,000 people over the age of 50 with no signs of cancer in combination with 25,000 people over the age of 40 who are showing symptoms.
The Grail multi-cancer early detection (MCED) test is an example of how next-generation DNA sequencing can help improve public health. Cancer is the leading cause of deaths worldwide accounting for ~10m in 2020 alone. Next-generation DNA sequencing is assisting in the development of effective new treatments and early diagnosis tools which will increase the cancer survival rate.
When it comes to cancer treatment, the earlier the tumour can be detected the higher the chance of treatment being successful. With this simple blood test, early detection could become much easier and result in a higher survival rate, when mass testing can be rolled out. This MCED test is an example of a liquid biopsy – a test that can detect the genetic material of a tumour in the bloodstream.
The MCED test is promising for two main reasons:
- Its accuracy;
- Its ability to detect a wide variety of cancers, some of which currently have no early detection tests.
Accuracy – Results from the latest trial, published in Annals of Oncology, show that the MCED test had a specificity of 99.5% and an overall sensitivity (accuracy) of 51.5% across all types and stages of cancer. In general, this gives a high false negative rate, with 48.5% of patients given a negative reading when they have cancer. On a more positive note, cancer was incorrectly identified (false positives) in only 0.5% of cases.
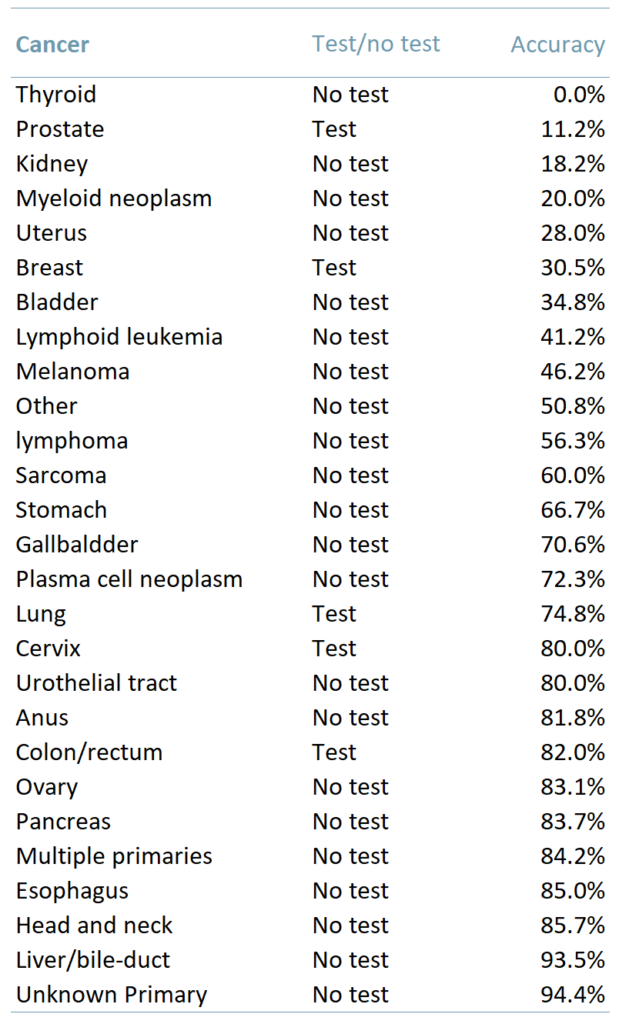
Exhibit 1 shows the results of this latest trial testing 4077 people. The table shows the type of cancer, whether there are accurate testing methods available and the percentage of cases in which the MCED test correctly identified the cancer.
Exhibit 1 – Accuracy of Grail’s MCED test depending on the type of cancer
 Sources: ACF Equity Research Graphics; Annals of Oncology
Sources: ACF Equity Research Graphics; Annals of Oncology
Ability to detect a wide variety of cancers – The MCED test can correctly identify >50 types of cancer. In addition, for solid tumours that currently do not have any screening options (oesophageal, liver, and pancreatic cancers) the ability to generate a positive test result was twice as high (65.6%) as that for solid tumours that do have screening options (breast, bowel, cervical and prostate).
In our assessment, MCED’s best use, given the false positive rate, is a combinatory approach with other established diagnosis tools – so it becomes part of the tool kit rather than replacing the entire bag. MCED is a positive development and, ceteris paribus, will allow clinicians to improve early detection of cancer in those who are most at risk between the ages of 50 and 79, and so too, survival rates.
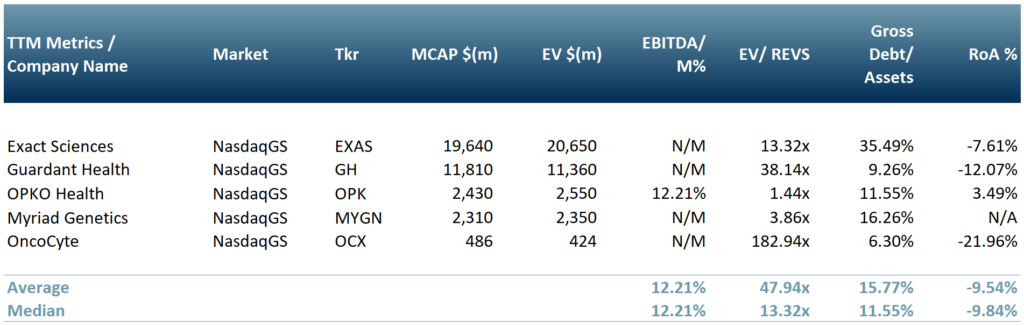
In exhibit 2 we have included a peer group table of companies working on diagnostics for the oncology sector. This includes Exact Sciences (NasdaqCM:EXAS), Guardant Health (NasdaqGS:GH), OPKO Health (NasdaqGS:OPK), Myriad Genetics (NasdaqGS:MYGN) and OncoCyte (NasdaqGS:OCX).
Exhibit 2 – Peer group table of companies working on diagnostics for the oncology sector
 Sources: ACF Equity Research Graphics; Refinitiv
Sources: ACF Equity Research Graphics; Refinitiv
At ACF, we predict the biotech/pharmaceuticals sectors will be the most disruptive over the coming decade. The advancements in gene/DNA sequencing now allows for sequencing of an entire human genome in one day vs a decade when the technology was first developed in the 1970’s.
As the technology has advanced it has also become cheaper meaning that companies with a smaller budget can now investigate its uses. This has the potential to dramatically improve public health and help overcome conditions and diseases that have plagued mankind for many years.
It is for this reason that we see large potential growth in investment for the sector and see biotech becoming the new “Big Tech” during the 2020’s, outperforming all other sectors including IT and data.
Author: Sam Butcher – Sam is a Junior Staff Analyst at ACF Equity Research. See Sam’s profile here
















