Omicron and Covid – immunology, evolution and economics
GSK’s C19 monoclonal antibody (mAb) treatment, sotrovimab, was approved by MHRA (UK medicines regulator) on 02 Dec 21. This treatment will be used on high-risk patients infected with omicron variant. In the meantime, BioNTech / Pfizer PFE mRNA vaccine was doing a roaring trade in London over the weekend.
- GlaxoSmithKline’s C19 antibody treatment is promising effective results against the Omicron coronavirus variant, according to early data. This is creating a little optimism following recent concerns of some pharmaceutical groups regarding the efficacy of monoclonal antidodies.
- GlaxoSmithKline, partnered with Vir Biotechnology in the development of sotrovimab.
- GSK’s treatment was approved for usage both in UK and US for high-risk patients, prior to the completion of the lab research on sotrovimab.
- There is a considerable difference between the action on a human of a vaccine vs. that of a monoclonal antibody treatment.
- Vaccines stimulate T-cells, B-cells and macrophages amongst other immune system cells.
- Whereas monoclonal antibody treatments focus only on the receptor binding domain on the spike protein of coronavirus.
- Monoclonal antibodies are laboratory made replicants of the antibodies produced by lymphocytes (i.e. T-cells and B-cells).
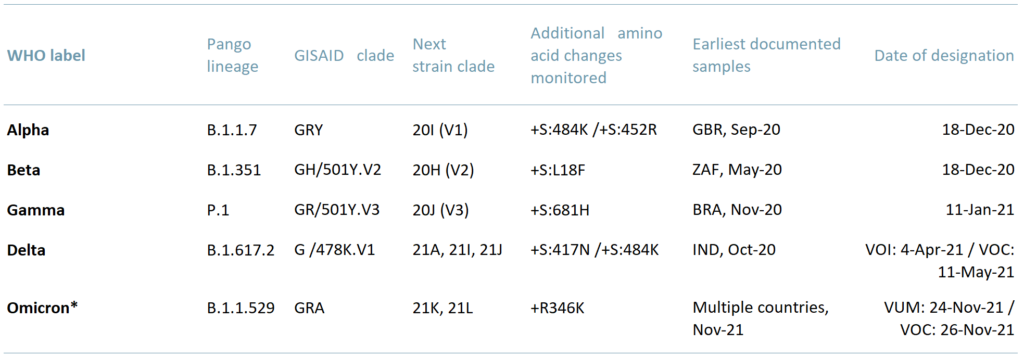
Exhibit 1 – Who data – named Covid variants and dates of designation
 Sources: ACF Equity Research Graphics; WHO
Sources: ACF Equity Research Graphics; WHO
In ACF’s view, every new C19 variant has the potential to disrupt economic growth. Virus genetic material replication systems are relatively unstable meaning errors are relatively frequent, which in turn leads to a faster evolution rate. Viral evolution rates far exceed the rate at which we can currently produce treatment protocols.
We infer based on WHO data that a variant of ‘concern’ is appearing/evolving approximately every 6-12 weeks. The WHO has so far classified 5 meaningful variants since December 2020.
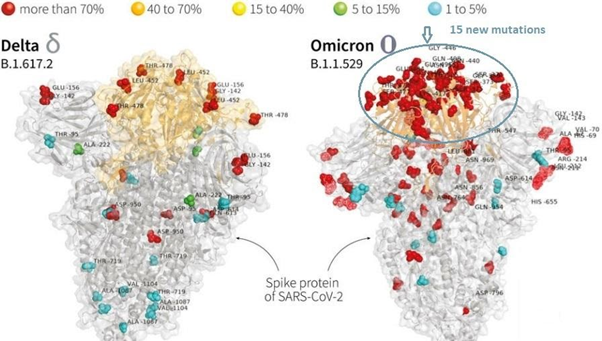
In exhibit 2 below we present two images that show just how different Covid-19 Delta and Omicron variants are where it matters.
Exhibit 2 – Spike Protein of SARS-CoV-2 – difference between Delta and Omicron variant
 Sources: ACF Equity Research; France24
Sources: ACF Equity Research; France24
In the Omicron variant there are 15 new mutations vs about 13 in the Delta variant in the receptor binding domain – the part of the spike protein that attaches itself to the human cell. According to Tulio de Oliveira, the director of South Africa’s Centre for Epidemic Response and Innovation, the difference between Omicron and the previous C-19 variants consists of a “very unusual constellation of mutations.”
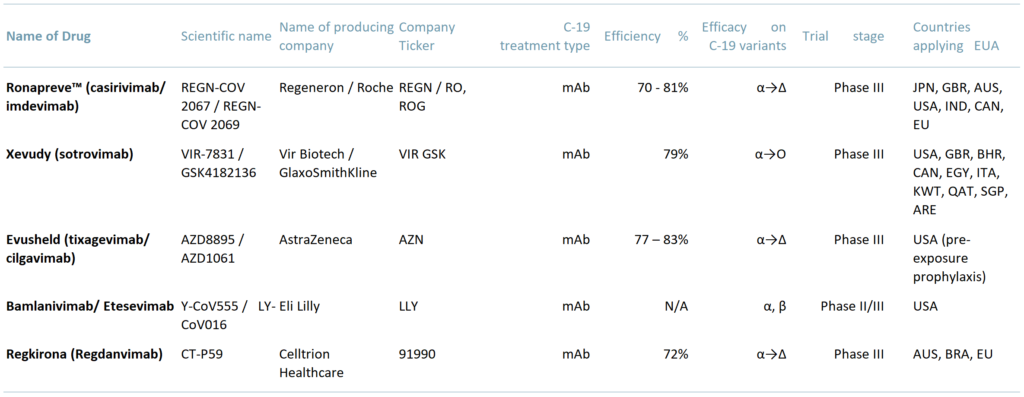
Apparently, some monoclonal antibody treatments are effective even against the Omicron variant. The sustainability of the existing antibody treatments is unclear though, as these drugs have been approved on initial effectiveness data only, from phase III (PIII) trials.
However, countries around the world are considering the Emergency Use Authorization (EUA) for most of the existing C-19 monoclonal antibody (mAb) treatments, while other countries have already applied it and are using a range of mAb – as per exhibit 2 below.
Exhibit 3 – Covid 19 antibody treatments – efficacy and approval
 Sources: ACF Equity Research graphics; Bioworld; Vir; Roche; FDA; AstraZeneca; Springer
Sources: ACF Equity Research graphics; Bioworld; Vir; Roche; FDA; AstraZeneca; Springer
Alternative types of C-19 treatments include Immunomodulators – for hospitalised patients – and oral antivirals. However, the best way to end a pandemic is to have a high percentage of the population vaccinated / immunised and to do this at a very high speed to reduce the probability of evolutionary changes in the virus (or bacteria).
Over the longer term, evolution is likely to be on our side – evolutionary pressure for any invading microorganism, bacteria or virus, drives it improve its transmission ease and rate, whilst keeping the host alive as long as possible – i.e. to be less and less debilitating to the host (in this case humans).
There is one slight fly in the ointment to the above general disease progression – if the first evolutionary pressure – transmission efficiency – leads to an extraordinarily transmissible strain of Covid, the second evolutionary pressure may never come to bear. The pressure for vaccine development, mAb and a generalised Sars-Cov-2 solution remains as high as ever.
For now global markets and the global economy are coping (more or less) and there is also some hope that a generalised Sars-Cov-2 treatment may be possible to create in the not too distant future – presumably using AI technology to speed the process.
Author: Anda Onu – Anda is part of ACF’s Sales & Strategy team. See Anda’s profile here
















